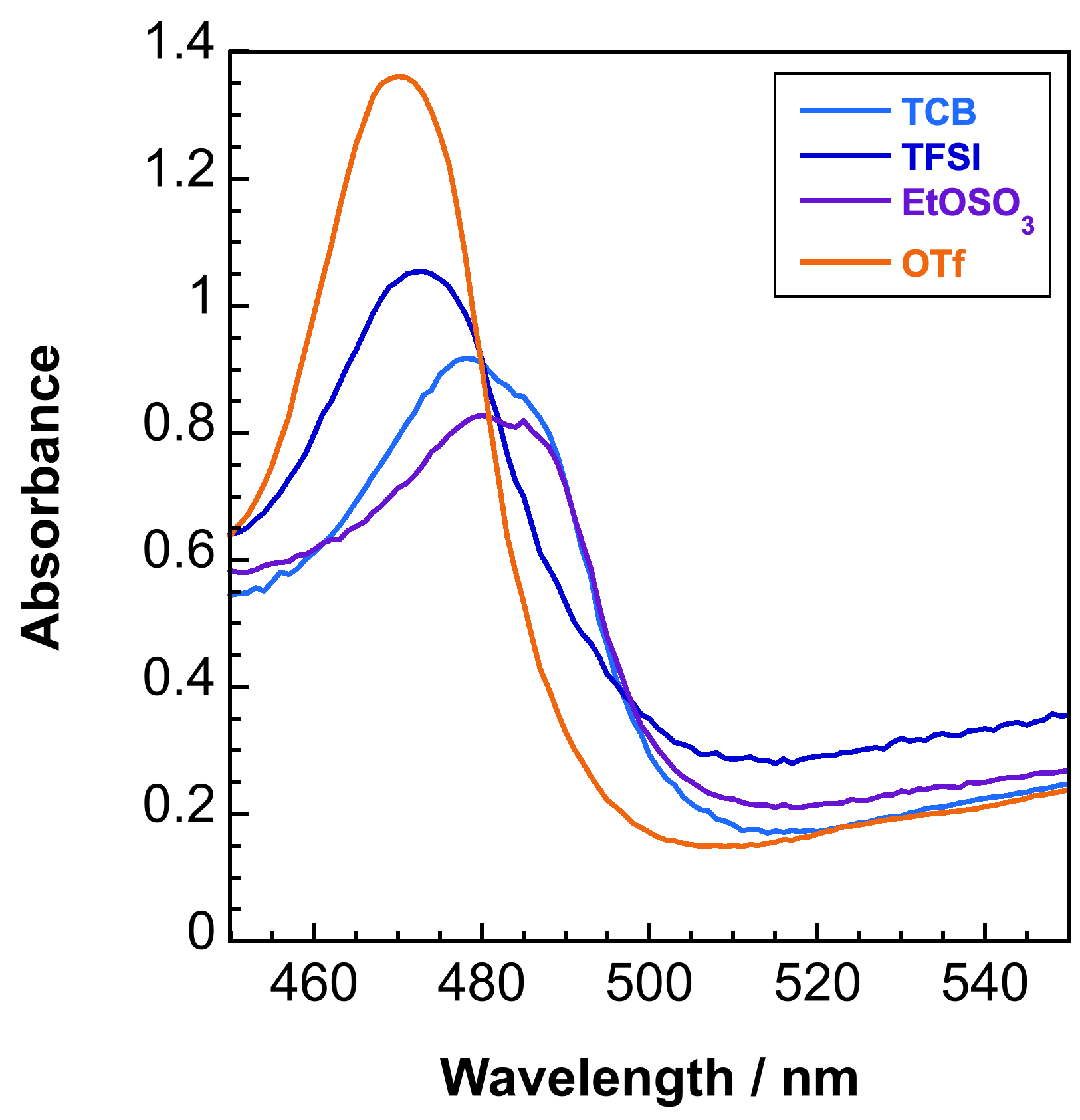
Ligand field and charge transfer transitions are critical to a variety of photochemical and electrochemical transformations. Using fundamental insight from a number of advanced spectroscopic and theoretical techniques (i.e. EAS/spectroelectrochemistry, EXAFS/XANES, MCD, TDDFT/CASSCF), we aim to identify key electronic transitions and structural properties that contribute to various modes of reactivity. We are presently interested in Lewis acid effects on mono-/multimetallic cyanide complexes and metalloporphyrinoids for applications in photo-/electrocatalysis and redox flow batteries.